Blog
How to Develop Research Questions Using PICOT Framework | 5 Step Guide in Evidence Based PICOT Question in Nursing and Clinical Studies
Understanding the PICOT Framework for Clinical Research
What is the PICOT Framework and Why is It Important?
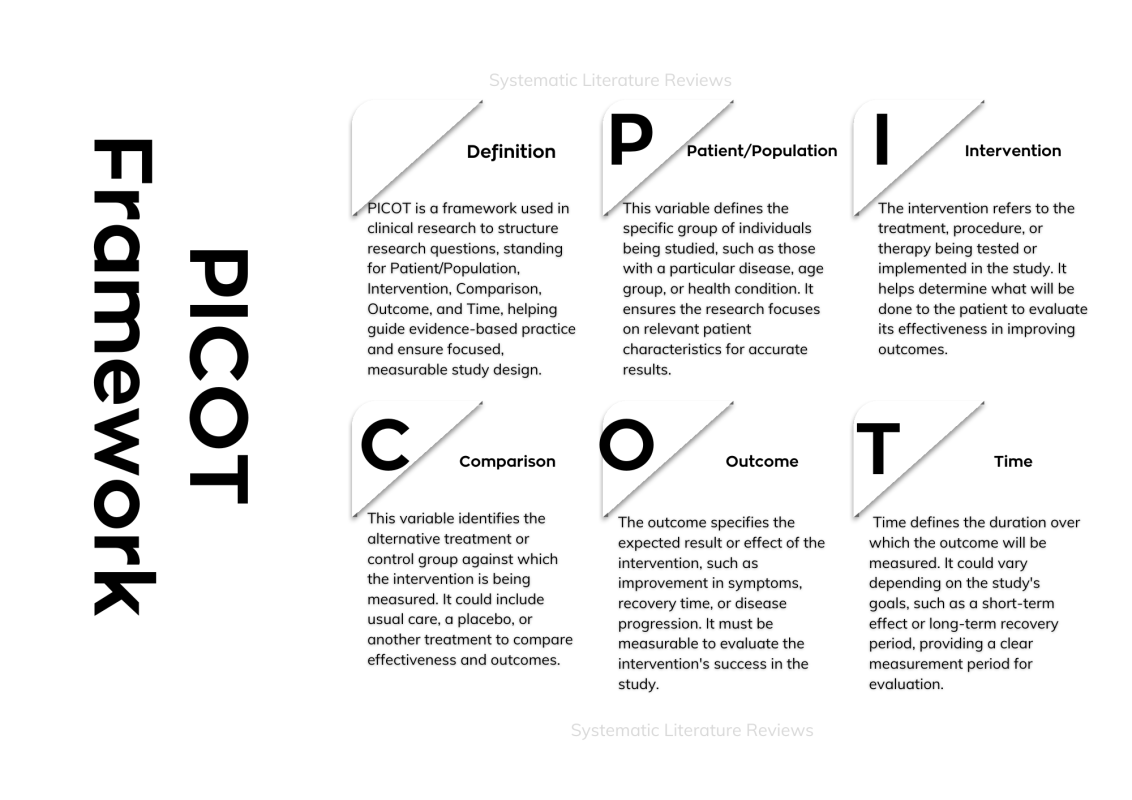
- PICOT Framework is a structured method for developing clinical research questions, particularly in evidence-based practice (EBP).
- It helps clinicians formulate specific, focused, and answerable research questions, leading to more effective patient care.
- The PICOT Framework is widely used in healthcare to evaluate treatment efficacy and determine the best practices for patient care.
- The PICOT acronym stands for Patient (or population), Intervention, Comparison, Outcome, and Time.
- By using the PICOT Framework, researchers can construct clear, focused questions related to patient care and develop their search strategy for literature.
- It guides clinicians to find the best evidence to inform clinical decisions and improve patient recovery.
Get Expert Systematic Review Help
Need assistance with writing a comprehensive Systematic Literature Review? Our experts can guide you through every step, ensuring high-quality, evidence-based analysis. Contact us today for professional support!
Key Components of the PICOT Format
- P (Patient/Population): Refers to the group of patients with specific diagnosis or health condition, e.g., patients with chronic diseases.
- I (Intervention): The treatment, procedure, or therapy being tested, e.g., a nurse-led intervention or a specific medicine.
- C (Comparison): An alternative treatment or usual care, or a placebo.
- O (Outcome): The expected result or effect, such as reduction in recovery time, improved health research outcomes, or increased treatment efficacy.
- T (Time): The period needed to observe the outcome, such as recovery time or time to diagnose the condition.
Defining Your Clinical Question Using PICOT
- Use the PICOT Framework to develop a clear foreground question that is measurable and related to the patient’s care.
- The PICOT Framework will help clinicians formulate evidence-based research questions by clearly defining the patient, intervention, and outcome of interest.
- Research guides and resources like PubMed and systematic reviews can provide the literature needed to answer the clinical question.
- A well-formulated PICOT question can aid researchers in evaluating the feasibility and validity of a proposed randomized controlled trial or other clinical studies.
- By constructing search strategies using keywords from the PICOT Framework, clinicians can effectively explore practice guidelines, epidemiology, and systematic reviews.
- This framework is crucial for clinically determining the most effective treatments and interventions for patient care based on high-quality, relevant evidence.
Get Quick Access to Other Key Research Question Frameworks Below
- Learn how to craft effective and achievable goals by reading the article on how to Write SMART Objectives | Best 5 Tips in Writing SMART Goals.
- Explore key tips on how to craft strong and focused research questions for successful outcomes in your studies by reading the article about Sample Research Questions | Tips for Good Research Questions.
- Discover how the PCC framework can help structure clear and effective research questions for your scoping review by reading the article about PCC Framework | Best for Scoping Review Research Questions.
- Learn how the SPIDER framework can guide you in formulating well-defined qualitative research questions by reading the article about SPIDER Framework | Formulate Qualitative Research Question.
- Understand how to use the PIO framework to create focused clinical research questions for your practice by reading the article about PIO Framework for Formulating Clinical Research Questions.
- Dive into the PEO framework to effectively formulate qualitative research questions that drive meaningful insights by reading the article about PEO Framework for Formulating Qualitative Research Questions.
- Find out how the PICO framework can enhance your ability to create evidence-based research questions for clinical research by reading the article about PICO Framework for Evidence-Based Research Question.
- Uncover the power of the SPICE framework in crafting systematic review research questions that yield comprehensive findings by reading the article about SPICE Framework for Systematic Review Research Questions.
Crafting a Strong PICOT Question for Evidence-Based Practice
How to Structure a PICOT Question Effectively in 5 Steps
- Step 1: Define the Patient/Population (P)
- Identify the patient group or population you are studying, considering factors such as disease, diagnosis, age, gender, or specific healthcare needs.
- Example: Patients with chronic diseases such as diabetes or hypertension.
- Step 2: Specify the Intervention (I)
- Determine the intervention or treatment you plan to study. This could be a medicine, nurse-led program, or a treatment technique.
- Example: A nurse-led intervention for improving medication adherence in diabetic patients.
- Step 3: Choose the Comparison (C)
- Define the comparison group or treatment. It may be a placebo, usual care, or an alternative treatment.
- Example: Usual care versus the nurse-led intervention.
- Step 4: Define the Outcome (O)
- Identify the expected outcome or result, ensuring it is measurable and related to patient care.
- Example: Reduction in recovery time or improved blood sugar levels in patients.
- Step 5: Set the Time Frame (T)
- Determine the period during which the outcome will be measured or observed. This could be the length of a study or the expected time for treatment effects.
- Example: Assessing treatment efficacy over a 6-month period.
Example of PICOT Research Question
- Research Question: In patients with chronic diabetes (P), does a nurse-led intervention (I) compared to usual care (C) result in a reduction in recovery time (O) over a period of 6 months (T)?
- This question is structured using the PICOT Framework, ensuring it is focused and answerable, helping the clinician to find the best evidence through systematic reviews and meta-analysis.
Choosing the Right Strategy to Formulate Your PICOT Question
- Search Strategy: Develop your search strategy by utilizing resources like the library at the university, PubMed, and research guides to identify relevant literature.
- Expertise: Seek guidance from clinicians or experts in the field to ensure that your question is aligned with clinical practice.
- Epidemiology: Consider relevant epidemiology data or practice guidelines when formulating your question to ensure its relevance to health research.
- Use keywords from your PICOT Framework question to ensure a precise and measurable search.
Common Mistakes When Creating a PICOT Question and How to Avoid Them
- Vague Outcomes: Ensure your outcome is clearly defined and measurable. Avoid vague terms like “improvement” or “better.” Be specific about the desired result, such as reduction in recovery time or improvement in treatment efficacy.
- Unfocused Population: Make sure your patient group is specific and relevant. For example, instead of just “patients,” specify the disease or condition, such as patients with chronic pain or hypertension.
- Unanswerable Questions: Avoid questions that are too broad or complex. Focus on answerable questions that are aligned with clinical practice and researcher interests.
- Overcomplicating the Comparison: Keep the comparison simple and directly related to your intervention, such as comparing a placebo to an actual treatment, rather than multiple interventions at once.
By following the PICOT Framework and these steps, you can effectively develop a strong and focused research question that will guide you toward finding the best evidence to improve patient care and health outcomes.
Here is an example of research question developed using PICOT Framework:
Research Question:
In adults with hypertension (P), does daily exercise (I) compared to no exercise (C) improve blood pressure control (O) over a 6-month period (T)?
| PICOT Component | Description |
| P (Patient/Population) | Adults with hypertension |
| I (Intervention) | Daily exercise |
| C (Comparison) | No exercise |
| O (Outcome) | Blood pressure control |
| T (Time) | 6-month period |
Practical Tips for Using the PICOT Framework in Clinical Research
Searching Databases for PICOT Questions and Clinical Research
- Utilize Medical Databases: Search databases like PubMed, Wilkins, and other health sciences resources to find relevant literature that answers your PICOT Framework question.
- Use Precise Keywords: Ensure your search terms align with the key components of your PICOT Framework—patient, intervention, comparison, outcome, and time.
- Reference Reliable Sources: Use guides to the medical literature and references from trusted sources to refine your search and find the best evidence.
- Screening for Relevance: When reviewing articles, ensure they fit the scope of your PICOT Framework question. Look for cross-sectional studies, randomized controlled trials, and other high-quality studies.
Understanding Levels of Evidence When Using PICOT Framework
- Identify the Level of Evidence: Use Guyatt, Meade, and Cleland’s guidelines to determine the level of evidence that fits your PICOT Framework question.
- Level 1: Systematic reviews and meta-analysis of randomized controlled trials (RCTs).
- Level 2: Single randomized controlled trial.
- Level 3: Cross-sectional studies or cohort studies.
- Level 4: Case-control studies and diagnostic tests.
- Level 5: Expert opinions or clinic guidelines.
- Implementing Evidence: Understanding the levels of evidence ensures you use the most reliable research to inform clinical decisions and implement evidence-based practice (EBP).
How to Tailor Your Search Strategy for Evidence-Based Clinical Practice
- Define Your Research Question: Use the PICOT Framework to narrow down your search question, making it more focused and answerable.
- Develop a Protocol: Create a search protocol to guide your search strategy, outlining the key terms, inclusion criteria, and databases to be used.
- Apply Filters: When searching, apply filters for primary care or specific diseases/conditions to ensure the research is relevant to your patient population and clinical setting.
- Ask the Right Questions: Tailor your question using the PICOT Framework to determine what kind of evidence you need (e.g., diagnostic tests, treatment outcomes, etc.).
Implementing the PICOT Framework in Study Design and Clinical Practice
Using PICOT Framework to Guide Your Study Design
- Guiding Research Design: The PICOT Framework helps clinicians and researchers structure study designs that are focused, measurable, and relevant to clinical practice.
- Establish Study Objectives: The PICOT Framework aids in formulating clear objectives for the study by identifying the population, intervention, comparison, and outcomes.
- Determine Methodology: Based on the PICOT Framework, choose the appropriate study design, such as a randomized controlled trial (RCT), cohort study, or cross-sectional study, to address the research question.
Defining the Key Variables in Your PICOT Framework
- Identify Key Variables: Within the PICOT Framework, define the main variables:
- Patient/Population (P): Define the patient group you are studying (e.g., patients with chronic disease).
- Intervention (I): Specify the treatment or intervention being tested (e.g., a new drug or therapy).
- Comparison (C): Identify any comparison group (e.g., placebo or usual care).
- Outcome (O): Clearly define the expected outcome, ensuring it is measurable (e.g., improved recovery rates).
- Time (T): Set a timeframe for measuring the outcome (e.g., 3 months).
- Clarify Measurement Criteria: Use the PICOT Framework to determine how each variable will be measured, such as using a specific scale or diagnostic tool.
Analyzing and Interpreting Data Based on Your PICOT Question
- Data Analysis: Once the study is completed, use the defined PICOT Framework variables to guide the analysis of the collected data.
- Use statistical methods to compare the outcomes in the intervention and comparison groups.
- Apply validity tests to ensure that the data supports the conclusions related to the PICOT question.
- Interpret Findings: Interpret the data by linking it back to the PICOT Framework question. Did the intervention produce the expected outcome? If so, how will these findings inform clinical practice and implementation?
- Make Recommendations: Based on the PICOT Framework and the study findings, develop practice guidelines or recommendations for future research or patient care improvements.
