Blog
Exploring Meta-Analysis in Medical Research: A Guide to Effective Study Design
Introduction to Meta-Analysis and Its Importance in Medical Research
What is Meta-Analysis and Why is it Essential in Medical Research?
- Meta-analysis is a statistical technique used to combine the results of multiple individual studies to draw more reliable conclusions about a specific research question.
- By synthesizing individual studies, meta-analysis enhances the statistical power of research findings, providing a more accurate effect size and overcoming limitations in sample size and variability across studies.
- It is particularly vital in medical research because it allows for a comprehensive view of the treatment effect across various populations, study designs, and methodologies, offering a broader understanding of clinical interventions.
- Meta-analysis of randomized controlled trials (RCTs) is considered the gold standard for evidence synthesis due to its ability to evaluate treatment effects with higher precision and reduce bias in meta-analyses.
Expert Meta-Analysis Writing Services
Experience accurate, high-quality meta-analysis with our professional writing services. Let our experts help you synthesize data effectively, ensuring reliable conclusions. Contact us today for tailored support in your research projects!
The Role of Systematic Review in Meta-Analysis
- A systematic review is the first step in conducting a meta-analysis, where researchers identify and evaluate all relevant empirical studies on a particular topic.
- Meta-analysis is essentially the quantitative component of a systematic review, which summarizes and synthesizes the findings of individual studies.
- The Cochrane Collaboration is renowned for producing comprehensive systematic reviews that include meta-analysis to assess the effectiveness of interventions in clinical research.
- The systematic review and meta-analysis process ensures that studies included are rigorously selected and analyzed to eliminate bias and provide more accurate research synthesis.
For Quick Access to Related Materials, Check Out These Meta-Analysis Resources
- Explore over 100 meta-analysis topics and systematic review examples to inspire your research in the article Best 100+ Meta-Analysis Topics | Systematic Review Examples.
- Learn how to develop a comprehensive meta-analysis protocol using PRISMA guidelines in the article Meta-Analysis Template | Systematic Review Protocol & PRISMA.
- Follow a practical 5-step guide to outlining and executing effective meta-analysis methodologies in the article Meta-Analysis Outline & Methodology | 5-Step Practical Guide.
- Gain insight from top meta-analysis examples written by expert review writers to enhance your own research in the article Meta-Analysis Example from Best Review Writers.
Common Pitfalls and Challenges in Meta-Analysis
- Heterogeneity in meta-analysis is one of the biggest challenges. Variations in study design, treatment effects, and sample populations across studies can affect the overall reliability of the meta-analysis result.
- Statistical methods like random-effects meta-analysis and sensitivity analyses are often employed to handle heterogeneity and assess the robustness of the findings.
- Bias in meta-analyses can occur if the studies included in the analysis are not representative of the broader research question. For example, publication bias may arise if positive results are more likely to be published, skewing the meta-analysis process.
- A forest plot is a common tool used in meta-analysis to visually represent the results of individual studies and the overall effect, helping to assess variability and consistency across included studies.
- Funnel plots are another visual tool used to detect publication bias and assess the quality of studies included in the meta-analysis.
- Network meta-analysis is a more advanced technique that allows for comparisons of treatments that have not been directly studied together, broadening the scope of meta-analysis and increasing its applicability in real-world settings.
By addressing these challenges and utilizing appropriate methods for meta-analysis, researchers can generate more accurate, reproducible, and reliable conclusions in clinical research and medical research overall.
Conducting a Robust Meta-Analysis: Methodologies and Best Practices
Statistical Analyses in Meta-Analysis: Approaches and Techniques
- Meta-analysis involves combining data from multiple studies to generate a summary statistic that reflects the overall treatment effect or association. The primary statistical methods used in meta-analysis include:
- Fixed-effects model: Assumes that all studies measure the same underlying effect, making it suitable for studies with similar populations and interventions.
- Random-effects model: Allows for variability in study results, recognizing that study populations may differ. This model is particularly useful when conducting a meta-analysis of clinical trials or observational studies where the effect varies across different research settings.
- Bayesian meta-analyses: A flexible approach that incorporates prior knowledge or beliefs about the effects. Bayesian methods are useful when limited data is available, such as in meta-analysis of rare diseases.
- Meta-analysis of odds ratios: A popular method for combining studies that report binary outcomes, providing insights into the likelihood of an event occurring across multiple studies.
- Meta-analysis of diagnostic test accuracy: Used to assess the diagnostic performance of tests across studies, incorporating sensitivity, specificity, and other relevant parameters.
- The Cochrane Handbook for Systematic Reviews outlines various methods of meta-analysis, providing guidelines for selecting the most appropriate statistical model based on the research question and data characteristics.
- The meta-analysis model may involve a random-effects model for meta-analysis if there is variability in study design and study population, ensuring more reliable results of a meta-analysis.
Sensitivity Analyses: Ensuring Reliable Results
- Sensitivity analyses are essential in meta-analysis to test the robustness of findings and ensure the reliability of results. These analyses help assess how changes in the inclusion criteria or statistical methods impact the conclusions.
- Exclusion of outlier studies: By systematically removing studies with extreme results, researchers can evaluate if the conclusions hold without their influence.
- Subgroup analysis: This method involves dividing studies based on certain characteristics (e.g., study design, sample size, or study population) to determine if the effect is consistent across subgroups.
- Sensitivity tests for publication bias: These tests, such as funnel plots, help identify whether the results are skewed due to selective publication of positive findings.
- Meta-analysis using different statistical models: Performing meta-analysis with fixed-effects and random-effects models allows for the evaluation of consistency in the overall meta-analysis estimate.
- Sensitivity analyses are critical to performing a comprehensive meta-analysis, ensuring that findings are not unduly influenced by a small number of studies or methodological flaws in the data.
| Methodology | Description and Application | Strengths and Limitations |
| Fixed-Effects Model | Assumes that the true effect size is the same across all studies and that differences are due to sampling error. Suitable for studies with similar study designs, populations, and interventions. | Strengths: – Simple and easy to implement – Provides precise estimates when study populations are homogeneous. Limitations: – Assumes homogeneity, which may not be realistic for diverse study populations – May lead to inaccurate conclusions if variability exists between studies. |
| Random-Effects Model | Assumes that the true effect size varies between studies due to differences in study populations and methods. Ideal for combining studies with diverse designs, populations, or interventions, such as in clinical trials or observational studies. | Strengths: – Accounts for heterogeneity across studies – Provides a more generalized estimate of effect sizes – More flexible and suitable for diverse studies. Limitations: – Can lead to wider confidence intervals – May underestimate precision when variability is small. |
| Bayesian Meta-Analysis | Uses prior knowledge or assumptions (priors) in combination with data to estimate the distribution of the true effect. Used when there is limited data or when combining information from multiple sources with different study designs. | Strengths: – Incorporates prior knowledge, useful for sparse data – Provides posterior probability distributions, offering more detailed insight. – Flexible and adaptable to various research questions. Limitations: – Requires subjective choice of priors – Computationally intensive – Results depend heavily on the chosen priors. |
Bias in Meta-Analyses: How to Identify and Minimize It
- Bias in meta-analyses can distort the conclusions, leading to inaccurate or misleading results. Identifying and minimizing bias is crucial to conducting a robust meta-analysis.
- Publication bias: Occurs when studies with positive or significant findings are more likely to be published, skewing the results of a meta-analysis. This can be assessed using funnel plots, which visualize the distribution of study results. If the plot shows asymmetry, it may indicate the presence of publication bias.
- Selection bias: Ensures that the studies included in the meta-analysis are representative of the population and research area. Proper selection criteria and a thorough literature review can help minimize this bias.
- Data reporting bias: Happens when only certain outcomes or subgroups are reported in the studies. The Cochrane Database of Systematic Reviews and the Preferred Reporting Items for Systematic Reviews (PRISMA) provide guidelines for transparent reporting, which can help address this issue.
- Random-effects meta-analysis: Using this method helps account for differences in study design, which may reduce the impact of bias in the analysis.
- Identifying and addressing biases through these strategies improves the integrity of the meta-analysis and ensures that the meta-analysis results reflect the true underlying effects.
Methods for Minimizing Bias in Meta-Analysis
- Comprehensive search strategies: A well-conducted literature review with multiple databases ensures that all relevant studies are included, reducing the likelihood of publication and selection bias.
- Inclusion of larger studies: Incorporating larger, well-designed studies may help balance out the effects of smaller, more biased studies.
- Use of quality assessment tools: Tools like the Cochrane Risk of Bias Tool are used to evaluate the quality of individual studies, ensuring that only high-quality studies are included in the meta-analysis.
- Regular updates and revisions: Given the ever-evolving nature of clinical and medical research, it is important to periodically update the meta-analysis with new studies to maintain its relevance and accuracy.
Meta-analysis is a powerful tool for synthesizing evidence in medical research. By employing rigorous statistical methods, conducting sensitivity analyses, and addressing potential biases, researchers can perform a meta-analysis that provides reliable and accurate conclusions for clinical practice and future research.
Improving the Accuracy of Meta-Analysis: Sample Size, Randomized Control Trials, and Statistical Considerations
Sample Size and Its Impact on Meta-Analysis Results
- Sample size plays a crucial role in the accuracy and reliability of meta-analysis results. The larger the sample size of individual studies, the more precise the overall meta-analysis estimate is likely to be.
- Increased statistical power: Larger sample sizes improve the statistical power of a meta-analysis, reducing the likelihood of type II errors (failing to detect a true effect).
- More precise effect size: With larger studies, the effect in meta-analysis becomes more reliable because the confidence intervals around the meta-analysis estimate tend to be narrower.
- Variability reduction: When smaller studies are included in a meta-analysis, they can increase heterogeneity across the studies, potentially affecting the meta-analysis results. Ensuring that larger studies are well-represented helps to minimize this.
- Meta-analysis of observational studies or clinical trials often face challenges related to sample size. Smaller studies can disproportionately influence the meta-analysis, making it crucial to perform random effects meta-analysis to account for variability across studies.
- While meta-analysis may be used to combine single studies of varying sizes, the influence of small studies can distort the overall findings. Incorporating studies with larger sample sizes generally leads to more reliable meta-analysis results.
The Importance of Randomized Control Trials in Meta-Analysis
- Randomized Control Trials (RCTs) are considered the gold standard in clinical research, and their inclusion in meta-analysis significantly enhances the robustness of findings.
- Higher quality evidence: RCTs are designed to minimize bias and confounding factors, making their inclusion in meta-analysis of controlled clinical trials a key factor in producing high-quality evidence.
- Minimization of bias: RCTs help eliminate many sources of bias, such as selection bias, because participants are randomly assigned to treatment or control groups. This strengthens the meta-analysis model, ensuring that the findings are reflective of the true effect in meta-analysis.
- Generalizability of findings: Including RCTs in systematic reviews of interventions or meta-analysis ensures that the results are applicable to a wide population. The use of meta-analysis in aggregating RCT results can provide definitive conclusions on treatment effectiveness.
- Statistical rigor: Random effects meta-analysis is often employed when combining results from RCTs in meta-analysis. This method accounts for the variability in study design and research populations, making it appropriate for synthesizing findings from different RCTs.
- Meta-analysis may not always be appropriate for observational studies or non-randomized trials, where biases can affect the meta-analysis results. Thus, including RCTs in the analysis strengthens the overall evidence.
Effective Approaches to Meta-Analysis: A Statistical Overview
- A meta-analysis involves several key statistical methods to ensure the accurate interpretation of combined study results.
- Fixed-effects vs. random-effects models: Depending on the study design, random effects meta-analysis may be preferred when there is heterogeneity in study design or study population. This model assumes that the study in the meta-analysis represents a sample from a larger population, allowing for the meta-analysis to account for variability across studies.
- Meta-analysis of odds ratios: When combining clinical trials and meta-analysis, the use of odds ratios can be particularly useful in assessing binary outcomes (e.g., treatment success or failure).
- Forest plots: A forest plot is a common visual tool in meta-analysis, helping to display the effect in meta-analysis for each individual study and the combined result. This graphical representation allows for easy assessment of the variability and consistency of findings across studies.
- Sensitivity analyses: A key component of meta-analysis methods, sensitivity analyses help assess the robustness of the meta-analysis results by evaluating how the inclusion of certain studies or data points influences the overall findings.
- Study quality assessment: Evaluating the quality of the studies included in a meta-analysis is critical to ensure that only the most reliable data are used. Tools like the Cochrane risk of bias tool are commonly employed in systematic reviews of interventions to assess the methodological rigor of individual studies.
- Meta-analysis may involve a comprehensive meta-analysis of clinical trials, or it could focus on a narrower research area, depending on the scope of the research. The method used will vary depending on the research question, meta-analysis methods, and available data.
The accuracy of meta-analysis can be significantly improved by considering sample size, the inclusion of high-quality randomized controlled trials, and employing appropriate statistical methods. When performed correctly, meta-analysis offers a powerful tool for synthesizing clinical evidence, enabling more reliable conclusions and guiding future research studies in medical research.
Get Accurate Meta-Analysis Reports
Need a comprehensive meta-analysis for your study? Our experienced writers provide precise, well-researched reports that bring clarity to your data. Reach out now to enhance your research with expert-driven insights!
Frequently Asked Questions | People Also Ask
What is a Meta-Analysis vs. Systematic Review?
- A meta-analysis is a statistical technique that combines the results of multiple studies to provide a single, more precise estimate of the overall effect of an intervention or treatment. It typically involves synthesizing data from randomized control trials, observational studies, and other research to calculate a summary statistic.
- Meta-analysis is based on the quantitative analysis of data from individual studies, while a systematic review is a more comprehensive approach that involves systematically searching, selecting, and evaluating previous research to assess the evidence on a particular topic.
- Systematic reviews may or may not include a meta-analysis. If a systematic review involves a quantitative synthesis, then it is a meta-analysis.
- Meta-analysis can also help identify patterns or variations in treatment effects across different studies, while systematic reviews of interventions often focus more on describing the characteristics and quality of the studies included.
| Aspect | Meta-Analysis | Systematic Review |
| Definition | A statistical method to combine results from multiple studies to obtain a summary statistic. | A comprehensive approach that synthesizes and evaluates the findings from multiple studies. |
| Purpose | To estimate the overall effect or treatment effect across studies. | To assess the quality, relevance, and findings of studies on a particular topic. |
| Quantitative vs. Qualitative | Primarily quantitative; produces a numerical summary (e.g., effect size). | Can be qualitative, focusing on study characteristics, or quantitative if it includes a meta-analysis. |
| Inclusion of Studies | Combines data from studies in meta-analysis that meet predefined criteria. | Includes studies that meet strict inclusion/exclusion criteria, but no statistical pooling is required. |
| Data Analysis | Involves statistical analysis (e.g., random effects or fixed effects models). | No statistical analysis; studies are summarized narratively or descriptively. |
| Focus | Focuses on research synthesis through quantitative data from included studies. | Focuses on reviewing and evaluating the overall body of evidence for a particular research question. |
| Outcome | Provides an overall summary statistic (e.g., odds ratio, mean difference). | Summarizes the findings and conclusions of the studies but does not provide a pooled estimate. |
| Application | Commonly used in clinical trials and medical research to guide practice and policy. | Used to identify gaps in research, assess the quality of evidence, and support clinical decision-making. |
What is a Meta-Analysis for Dummies?
- A meta-analysis is simply a method used to combine results from different studies to get a clearer, more reliable conclusion about a specific research question or hypothesis.
- Think of it as looking at previous research as pieces of a puzzle. Each study in the meta-analysis provides a small piece, and by combining them, you get the full picture.
- In meta-analysis, researchers calculate the overall effect by considering how studies in meta-analysis report the same treatment or intervention outcome, then aggregating the results.
- The method for the meta-analysis involves careful selection of studies, ensuring that the studies are relevant, have adequate data, and are methodologically sound.
- Meta-analysis is performed using statistical techniques to ensure that the pooled results reflect the best available evidence, which helps guide clinical decisions or future directions for future research.
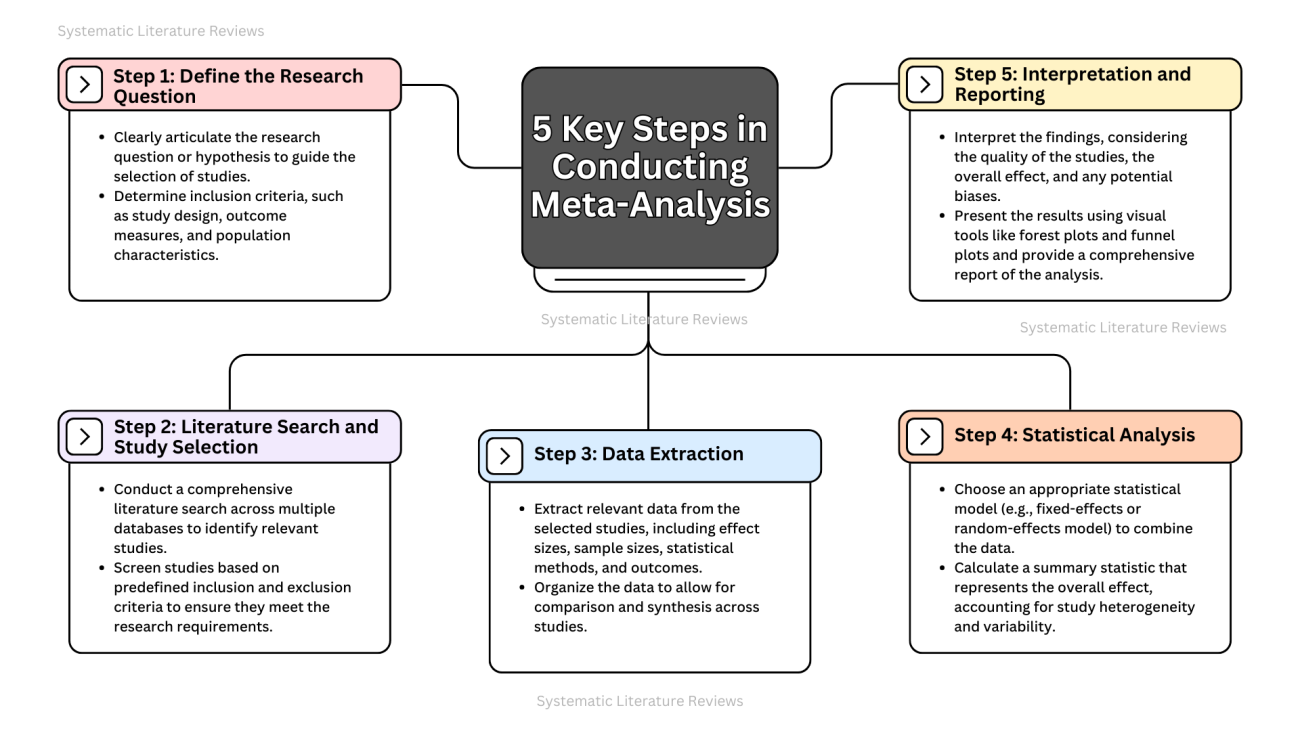
What Are the Four Basic Steps of a Meta-Analysis?
- Define the research question: Clearly identifying the research question is essential for determining which studies to include in the meta-analysis.
- Literature search: A systematic search of the literature to identify studies in meta-analysis that meet predefined criteria (e.g., study design, sample size, outcome measures).
- Data extraction: Extracting relevant data from the selected studies, such as effect sizes, statistical information, and methodological details.
- Statistical analysis: Conducting the meta-analysis using appropriate statistical models, such as random effects meta-analysis or fixed effects models, to combine data and produce a summary statistic or overall estimate of the treatment effect.
How to Tell if Something is a Meta-Analysis?
- To identify if a study is a meta-analysis, look for the following:
- Inclusion of multiple studies: Meta-analysis involves the combination of results from multiple independent studies.
- Quantitative synthesis: Unlike a systematic review, which may describe the findings of studies qualitatively, a meta-analysis performs a statistical analysis to combine data and present an overall result.
- Clear methodology: A meta-analysis will outline the method for the meta-analysis, including how studies were selected, the inclusion criteria, and how data were pooled or aggregated.
- Summary statistic: Meta-analysis is based on calculating an overall effect size (e.g., odds ratio, mean difference) from the data extracted from each study.
- Meta-analysis model involves techniques like random effects models for meta-analysis or fixed effects models, which adjust for variability in study results and populations.
Meta-analysis and systematic reviews are related but distinct methods for synthesizing evidence. While systematic reviews provide a comprehensive overview of research, meta-analysis offers a more quantitative approach to combining results. Understanding the promise and problems of meta-analysis, including its limitations (e.g., meta-analysis cannot always account for biases), can help researchers and clinicians apply its findings more effectively.
Quick Links to Meta-Analysis Resources – Learn More
- Explore over 100 meta-analysis topics and systematic review examples to inspire your research in the article Best 100+ Meta-Analysis Topics | Systematic Review Examples.
- Learn how to develop a comprehensive meta-analysis protocol using PRISMA guidelines in the article Meta-Analysis Template | Systematic Review Protocol & PRISMA.
- Follow a practical 5-step guide to outlining and executing effective meta-analysis methodologies in the article Meta-Analysis Outline & Methodology | 5-Step Practical Guide.
- Gain insight from top meta-analysis examples written by expert review writers to enhance your own research in the article Meta-Analysis Example from Best Review Writers.
